Damage Mechanisms and Identification
Formation damage can be referred to as the mechanism that alters the permeability of the near wellbore region, causing loss of injectivity performance. It has proved to be a major challenge to predict when it is expected, what mechanisms will be involved and what effects the damage will cause. There are many factors that contribute to the formation damage mechanisms. Still, it is not fully known which factors are involved, what effect each one of them has and how they interact with each other. The most general factors involved are believed to be:
-
Flow rate and pressure
-
Formation characteristics
- Fluid characteristics
The most common form of the formation damage, as identified
by researchers:
- Particle invasion
- Oil droplet invasion
- Invasion of hydrocarbon “solids”, such as emulsion, wax
and “schmoo”
- Scale deposition
- Corrosion
- Bacterial growth
- Formation fracturing
The effects of these factors are various, many of them
interact with each other and many are not considered a major problem in
relation to injection of produced water and some can even in some instances have great advantages.
Particle Invasion
Many researchers have studied invasion and formation
plugging of small particles in the injection fluid, small enough to flow
through the intergranular pore space.
Sources of Particles
The particles causing formation plugging can either be
“fines” loose in the formation pores, as described later, or transported with
the injected produced water. The latter are of many different types sources, such as:
- Formation particles from the formation water
- Precipitated ions from the formation or sea water
- Iron particles either corroded or eroded from the
casing, tubing, flow lines or other equipment in direct contact to the
injection water
- Scaling precipitates
Particle Plugging Mechanisms
Plugging or bridging due to invasion into the formation of
solid particles suspended in the injected water is traditionally the most
common explanation for reduced injectivity. Extensive laboratory work has been done in an attempt to
gain understanding of the phenomenon to be able to predict the required water
quality for injection wells. These observations confirm that small micron size particles, either loose within the formation pores or suspended in injection water, may cause considerable decline
in injectivity.
The various investigators studying the issue of particle
capturing associated with water injection have given almost as many
descriptions on the mechanisms involved. In 1972, Barkman and Davidson of Shell, suggested that impairment from suspended solids occurred by one or more of four mechanisms [Barkman, 1972
]. Since then, their description has been widely recognised. More recently, Roque et al. of Elf and Institut Français du Pétrole in France, explained the retention of solids suspended in injection water as consisting in four overlapping phases [Roque, 1995 ]. These two descriptions are listed in the table below.
Table 1. Descriptions of Formation Damage Mechanisms
|
Barkman and Davidson (Shell), 1972
|
Roque et al. (IFP), 1995
|
| Wellbore Narrowing: The solids from a filter cake on the face of the
wellbore. |
Deposition onto grain and pore throat surfaces. |
| Invasion: The solids invade the formation, bridge and form an internal filter cake. |
Formation of mono- or multi-particle bridges across pore throats,
followed by retention upstream from the bridges. |
| Perforation Plugging: The solids become lodged in the perforations. |
Internal cake building when non-percolating threshold is reached near
the entrance of porous medium. |
| Wellbore Fill-up: The solids settle to the bottom of the well by gravity and decrease the net zone height. |
External cake formation.
|
The following is a brief description of the formation damage mechanisms, as described by Roque et al., 1995.
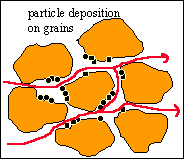
Surface Deposition
In this phase, the kinetics of this deposition is strongly
anisotropic. The effect of particle deposition on permeability is significant
only if it takes place in pore throats. Thus permeability reduction is not
related to the total amount deposited but only to the fraction deposited in
pore throat area.
|
|
|
|
|
|
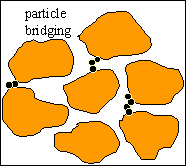
Pore Bridging
Once a particle is flowing through a pore throat it may
get attached to one or more particles already deposited onto the pore throat
surface, forming a bridge. Once a bridge is formed and consolidated, the
newly arriving particles accumulate upstream from bridged pores, thus
decreasing drastically fluid flow rate through these pores. |
|
|
|
|
|
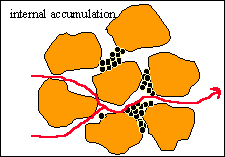
Internal Cake Formation
When the fraction of bridged pore throats reaches a critical
value, the pores are no longer connected over some critical characteristic
depth (damage depth). Then all the incoming particles accumulate not only
upstream from the bridged pore throats but also inside all pore bodies still
accessible, forming what is called internal cake. |
|
|
|
|
 |
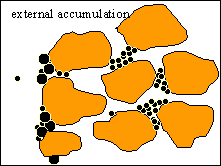
External Cake Building
As soon as internal cake formation is achieved, particles
accumulate upstream from the inlet of the porous medium, thus forming the
external cake. External cake may also be formed by particles which are bigger
then the average pore throat size. |
|
|
|
|
 |
Laboratory and field observations confirmed the theoretical
prediction that small micron size particles suspended in injection waters may
cause severe injectivity decline. In contrast with large particles that form a
remediable external filter cake, small particles can penetrate at large
distances from injection wells (internal accumulation) where remediation is
questionable. The bridging of pore throats is strongly dependent on the effective pore throat-to-particle size ratio, and the pore-throat size is often reduced by previous surface
deposition.
Fines Migration and Sanding
Muecke et al. demonstrated with wide variety of microscopic
photographs that all natural porous materials contain small mobile solids that
uniformly cover the interstitial solid surface of the pore space. These
particles are classified as “fines” and are either deposited over geological
time or introduced during drilling and completion. Studies on number of
sandstone cores in the U.S. Gulf of Mexico showed that typically these fines
are (Muecke):
- Quartz 39 wt%
- Amorphous materials (substances without crystal structure) 32 wt%
- Clay 11 wt%
- Other minerals (feldspars, muscovite, calcite, dolomite
and barite) 18 wt %.
If the fluid saturating the pores is set in motion, e.g.
during waterflooding (in water wet reservoirs) these fines can be entrained,
and subsequently are either carried all the way through the formation by
produced fluids or redeposited at preferred accumulation sites, causing pore
restrictions and large reduction in permeability. The trapping mechanism can be
simple bridging, flocculation or coagulation. Once bridges are formed they act
as increasingly effective trapping the particles that follow, e.g. solids in
the injected fluid.
Muecke identified several mechanisms affecting the particle
migration, such as particle size, concentration, velocity and interactions of
the wettability of the fines and the injected fluid. He discovered that the tendency for bridging is related to
concentration but the bridge stability is related to the flow velocity when the
bridging occurs. Bridges formed at low rate can be easily broken by varying or reversing the flow or by pressure pulses but bridges formed at higher rate can be very stable.
Gruesbeck and Collins showed that even though fines can be
mobilised at very low velocities, they don’t get trapped until the flow
velocity has reached a certain critical limit. This critical velocity depends on water salinity, but for corefloods in sandstone with 2-3% KCl, this velocity has been reported to be around 5x10-5 m/s (BP REPORT).
Many researchers have observed fine particles unsuspended
within the formation pores [Gruesbeck, 1982], Muecke and others. If the pressure difference is increased
by injection, these fines are free to move until they reach equilibrium again,
i.e. are re-deposited. During that
process, they can plug the pore throats by one or more of the plugging
mechanisms described before.
Most researchers assume particles of sub-micron sizes flow
through the formation with the injected fluid without being captured. Others claim that this is not
necessarily the case, sub-micron particles can also be captured by the same
means as the larger particles as well as by van der Waals-type electronic
forces [Vetter, 1987].
Oil Droplets Invasion
A special case of “fines” migration is “sanding” of
unconsolidated sandstone formations (soft sands). These are dealt with specifically in Task 3.
The stream of produced water will always contain oil even
though it has been carefully treated by a free water knock-out drum tanks or
modern hydrocyclones. The oil is in the form of:
- Free oil
- Emulsified oil
- Dissolved oil
|
|
|
|
|
|
Core flood experiments have shown that the first two can
cause permeability damage and the two main processes believed to be involved
are:
- Droplet pore blocking due to surface and interfacial
forces
- Wettability changes, causing changes in relative
permeability
The free oil can cause a reduction in relative permeability
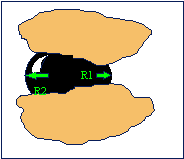
to water where as emulsions can act as a pore throat blocking agent. Coleman and McLelland have shown that
for an emulsion to be an effective blocking agent, the oil droplet size should
be slightly larger than the pore throat size, as can be seen on the picture on
the right [Coleman, 1994].
In core tests made during a design of PWRI scheme below
fracture gradient for Shell Nigeria, it was claimed that the greatest
contributor to skin damage was resulting from the combination of fines
migration and emulsion block in the near wellbore area. Using solvents decreased the
injectivity loss of 40-50% [Ohen et al. 1996].
The effects of dissolved oil are not as well
understood. Some researchers even
claim it has no impact on injectivity [Coleman, 1994].
Pore Blocking of Droplets
Results of core studies indicate that injection of oily
water can cause formation permeability to decline and that droplet size can
have a greater effect on the permeability decline than oil concentration. In the experiments reported by Todd et al., a dispersion containing 100 ppm crude oil, having a mean diameter of 7.09 mm caused more severe permeability damage to core plugs with a median pore radius
of 12.3 mm than another 500 ppm crude oil concentration dispersion having a mean diameter of 3.43 mm. The average permeability decline rate
for the former dispersion injected was 2.83% per pore volume injected. The
dispersion with 500 ppm and 3.43 mm droplet size injected had a permeability decline rate of only 1.14% per pore volume injected.
Permeability Changes due to Wettability and Surface /Interfacial Forces
Relative permeability is a measure of the ability of the formation to conduct one fluid in the presence of one or more other miscible fluid types. Long-term injection
of produced water with oil content of 100 ppm could leave behind a residual oil
bank. This could reduce the relative permeability to water and introduce the tendency of other suspended
material to be entrained near the wellbore. The result would be injectivity loss due to the formation of
a stable emulsion. Ohen et al. studied the increase of oil saturation in the near welbore area as a function
of time by injecting synthetic produced water with 100 ppm OIW into cores [Ohen et. al 1996]. After injection of about 5000 pore volumes 10% residual oil saturation had been created and permeability to water
had reduced by 70%.
Hydrocarbon Deposition
Not much has been dealt with deposition of hydrocarbon
materials in injectors, else than emulsions or free or dissolved oil and these
are usually not considered as severe damage mechanisms. Still, reporting on three different
mechanisms can be found, mainly where “heavy” hydrocarbons are present:
- Wax deposition
- Organic deposits
Wax Deposition
King and Adegbesan reported in-situ wax precipitation
associated with particle deposition in injection wells in the Pembina Cardium
reservoir in Canada, causing injectivity decline (King, R.W. and Adegbesan,
K.O.: “Resolution of the principal formation damage mechanisms causing
injectivity and productivity impairment in the Pembina Cardium reservoir.”)
[King, 1997].
Organic Deposits
Organic deposits are heavy hydrocarbons (paraffins or
asphaltenes) that precipitate as the pressure or temperature is reduced. They are typically located in the
tubing, perforations or formation. Although the formation mechanisms of organic deposits are numerous and
complex, the main mechanism is a change in temperature or pressure in the flow
system. Cooling of the wellbore due to injection of cold water has a much more pronounced effect.
Scaling
Scales are water-soluble chemicals that precipitate out of
solution in response to changes in conditions. Scaling is usually caused by incompatibility of injected and
formation waters. The formation of scale and subsequent plugging of injection
wells can present serious problems in water injection, particularly where PWRI
is concerned. Scaling in production systems has been a fairly common experience
in the North Sea. The most common types of scale are calcium carbonate, calcium
sulphate, barium sulphate, strontium sulphate and iron complexes. Reservoir
scaling is not a serious concern in seawater injection schemes. Production well
and systems scaling is the major concern and PWRI systems will be similar.
Injection well scaling with PWRI may be more of a concern as
a result of the release of dissolved gases and the downhole temperature effect.
Where produced water is re-injected as part of a waterflood
operation, the volumes produced will not be sufficient for pressure maintenance
purposes, even when significant water breakthrough has occurred. Thus an
additional water source will be required unless the reservoir is allowed to
deplete. Mixing of sea water and formation water usually causes various
insoluble salts to form and precipitate, also causing scaling of the reservoir
and production system. In the Pembina Cardium field, analysis of scale samples
from an injection well was reported to contain a very high iron content,
probably corrosion material from the injection equipment and tubing [King,
1997].
Calcium Carbonate
Calcium Carbonate is by far the most common form of scale
encountered. Calcium carbonate scale results from the following reaction:
Ca2+ +
2(HCO3-) = CaCo3 +H2O
+ CO2
Precipitation can occur in systems where the operating pressure is insufficient to keep the carbon dioxide in solution. Pressure drops and turbulence commonly occur downstream of
restrictions in the production string and surface production system. With PWRI,
further precipitation may occur in injection wells due to increased temperature
and the fact that a portion of any CO2 present will have been
separated during topside treatment.
Calcium Sulphate
Calcium Sulphate forms the second most prevalent type of
scale generally formed in injection wells. It may occur in three forms:
-
gypsum, CaSO4 . 2H2O
- anhydrite, CaSO4
- Plaster of Paris, 2CaSO4 . H2O
The pressure and temperature of the system determine the
form of calcium sulphate precipitated. Gypsum solubility is much greater than
calcium carbonate. Thus, a water containing sulphate, carbonate ions and
calcium ions will precipitate calcium carbonate first. However the solubility
of calcium sulphate becomes less at high temperatures and some of the deeper hotter
fields may encounter this scale. Calcium sulphate scales are found in the
shallower tubing and surface equipment, where the pressure and temperature are
lower than downhole. But they can also occur in the reservoir at temperatures
higher than 110°C.
Barium Sulphate and Strontium Sulphate
Barium and strontium sulphate occurrences are not as common
as the two calcium scales mentioned previously. However, some reservoirs
contain significant quantities of both barium and strontium ions. When there is
mixing between sea water and formation water, such as occurs when injected sea
water breaks through at a production well, then sulphate scales will
precipitate. Barium sulphate scale can be the most critical type of scale
deposit in surface and downhole equipment in oil wells. Since it has the lowest
solubility of the sulphates in the mixed water, it precipitates most easily.
Hence it can form in the tubing as the fluids rise to the surface. It is not
soluble in hydrochloric acid, but can be dissolved with chemicals such as
Ethylene Diamine Tetra Acetic Acid (EDTA). Strontium sulphate is more soluble
than barium sulphate and hence precipitates more slowly. However, it is still
likely to form in association with barium sulphate in the production string,
near and on surface and particularly in settling vessels.
Corrosion
Corrosion results from the
chemical interaction of the steel with various gasses and fluids found in the
down-hole environment leading to the formation of iron sulfide and iron oxide
both of which are reactive with acids and other chemicals. Corrosion can also
arise from the steel coming in contact with the acid itself. Reaction between
water and CO2 can lead corrosion problems. Presence of H2S
accelerates the corrosion rates. Common problems associated with iron sulfide
are plugging of equipment, filters, injection lines and the formation
face. Solution to corrosion mitigation includes water treatment with acrolein [Salma, 2000], biocide
[Rosser et al., 1999], sulfur dioxide to scavenge dissolved oxygen. [Nassivera
and Essel, 1979; Meyers et al., 1977] to use corrosion resistance steel or
alloys [Chitwood and Coyle, 1994; Nielsen and Bingham, 1998].
Bacteria Growth
The role of bacteria in produced water systems and their
contribution to plugging, souring and corrosion can be a great concern.
Microorganisms can be a source of plugging in much the same way as sand and
other agents. Three types of bacteria are of concern in produced water, namely:
- Sulphate-reducing bacteria
- Slime-forming bacteria
- Iron bacteria
- Sulphate-Reducing
Bacteria (SRB)
Sulphate reducers probably cause more serious problems in
oilfield injection systems than any other bacteria. They occur in such
quantities that they do not always constitute a menace by plugging with their
physical bodies. However, they are
of great concern because they induce corrosion and pitting, produce H2S, which
will increase the corrosivity of the water, and form iron sulphide solids that
may plug injection wells.
The production of H2S occurs by the following mechanism:
SO42- + 8(H) = H2S + 2 H2O +2 OH-
SRB prefer a neutral pH environment. In the laboratory
activity is observed within a pH range of about 5.5 to 8.5. However, in more
acidic conditions, the metabolic products of SRB represent buffers namely the
HS- H2S and the HCO3-.
Corrosion of surface and downhole equipment has been
associated with sulphate-reducing bacteria. It induces iron sulphide solids
that, if they become free and are transported to the sandface, could have a
plugging effect.
Slime Forming Bacteria
Slime forming bacteria can cause formation damage in water
injection wells. These bacteria attach to solid surfaces by a “glycocalyx” film
in nature, but do not manufacture this substance in laboratory cultures.
Glycocalyx or biofilm layer is a tangled mass of polysaccharide fibres that
extend from the individual bacteria cells or colony of cells to allow the
bacteria to adhere to surfaces in the oil production system and to rock
formations. This film may cover or trap the Sulphate-Reducing Bacteria (SRB).
The SRB growth within the biofilm can also generate H2S gas that reacts with
dissolved iron to precipitate iron sulphide, increasing solid particle loading
in the injection water. The belief is that the glycocalyx is not required in
laboratory cultures because there are no forces acting against the bacteria, as
there are in a flowing water stream.
Bacteria can produce exopolysachcarides that coalesce to
form a confluent biofilm. Oil and solid particles entrained in the injection
water can also be trapped by the developing bacterial biofilm to significantly
accelerate water injectivity decline rates.
Biofilm growth in surface facilities can interfere with
separator and gas flotation cell performance, resulting in increased oil-in-water
carryovers. Additionally, underneath the biofilm, corrosion can occur.
Iron Bacteria
Iron bacteria deposit a sheet of iron hydroxide around them
as they grow. The iron bacteria are obtained from soluble iron ions in the
water. They are classified as aerobic bacteria, although they can apparently
grow well with only trace amounts of oxygen. Iron bacteria can cause both
corrosion and plugging. Although they do not directly participate in the
corrosion reaction, corrosion can either result from the activity of sulphate
reducers under the hydroxide sheet or by the creation of an oxygen concentrated
cell.
Damage Identification
The objective of damage identification is to aid selection of
the optimum treatment or stimulation design. To achieve this objective, certain laboratory tests must be
performed. These tests may include:
-
Core Analysis
- Formation Mineralogy Analysis
- Formation Wettability
- Petrophysical Characterization
- Formation fluid and water analysis
Core Analysis
The detailed analysis of
formation cores is required to design the damage removal treatment. It is
difficult to determine formation mineralogy without the use of cores (sidewall
or conventional). Conventional
cores are recommended to complete the analysis because sidewall cores can be
contaminated with drilling fluids and may not be representative of the
formation. If sidewall cores are used, the analysis should be conducted on
duplicate cores.
Formation Mineralogy
The formation mineralogy is
an important parameter affecting stimulation success. Knowledge of the
petrography of the formation is essential to understanding what the response of
the rock (formation material) will be to any fluid. The relation between the
rock and the treating fluid depends on the minerals present and the position of
the minerals within the rock matrix. The analytical techniques used to
characterize the mineralogy are X-ray diffraction (XRD), SEM and thin-section
analysis. XRD analysis provides rapid and accurate identification of the
crystalline material of the rock matrix. SEM provides information on mineralogy and morphology
and the size of pore-lining materials. Thin-section analysis is used widely to study rock structure and
quantify minerals.
Formation Wettability
Most formations (sandstone
or carbonates) are water-wet. Occasionally, oil-wet formations are
encountered. The simplest test to determine formation wettability is to take approximately 10 cm3 of formation material and place it in the produced brine to equilibrate for
approximately 30 min. The formation material is then placed in an oil (such as
kerosene) and observed. To accentuate the test results, red dye can be added to
the clear oil to aid identification of the oil adhering to the formation
material. After it is allowed to equilibrate for an additional 30 min, the formation material is added to a
fresh aqueous solution. Strongly water-wet formations or other fines disperse
readily in aqueous fluids but agglomerate or clump together in the clear
oil-base fluids. Conversely, oil-wet particles disperse in oil but agglomerate
in water-base fluids. The surface is water-wet if the contact angle of the
fluid with the formation material is less than 90°; the surface is oil-wet if
the contact angle is greater than or equal to 90°. Wettability can exist in various degrees between extremely water-wet and extremely oil-wet. Intermediate wettability is difficult to
identify and describe, with contact angles greater than 80° but less than 100°.
The wettability test can also be used to determine if the desired treatment
fluid is water-wetting or oil-wetting and how the treatment fluid may affect
the desired natural wettability.
Petrophysical Characterization
Core porosity and
permeability should be measured before performing a core flow evaluation. The porosity of the rock sample can be determined using one of several techniques. The simplest technique for the
determination of effective porosity uses Boyle’s law; the pressure of nitrogen
is determined in a constant-volume cell, with and without the core. The total
porosity is derived by bulk and matrix density measurements with a helium
pycnometer. When required, the pore-size distribution can also be measured
using a mercury intrusion porosimeter.
Permeability, an intrinsic
characteristic of the rock, is a measure of the rock’s capacity to transmit
fluids. The measurement is usually made with gas (e.g., nitrogen [N2]) or
liquids (e.g., brines and oils). Permeabilities must be determined using simulated downhole temperature
and stress conditions, especially for stress-sensitive formations such as soft
sediments.
Formation Fluid and Produced Water Analysis
Analysis of the formation
brine and oil can aid in determining the types of damage that may be
present. Analysis of the formation
brine can be used to predict scale formation. CaCO3 is usually formed when the pressure is
reduced on waters that are rich in calcium and bicarbonate ions. Iron scales such as iron carbonate and
iron sulfide can be extremely difficult to remove. They are usually seen in
wells that have both a high back-groundiron count and a tendency to precipitate
calcium carbonate. Iron sulfide scales react according to their structure.
Seven different forms of iron sulfide scale have been identified. Only two of
these iron sulfide forms are readily soluble in hydrochloric acid (HCl). The
remaining iron sulfide scales are either slowly soluble or not significantly
soluble.
Chloride scales, such as
sodium chloride precipitation from water caused by temperature decrease or
evaporation of the water, are common. There is no effective way to prevent salt
precipitation, and salt has a limited solubility in acid.
If the injected fluids are
incompatible with the oil, formation of emulsions can occur. The oil may also
contain paraffins and asphaltenes. The quantity of various fractions of
asphaltenes and paraffins and their ratio to each other are used to assess the
possibility of organic precipitation damage.
References
- Abrams, A.; Mud Design to Minimize Rock Impairment
due to Particle Invasion. JPT
(1977).
- Barkman,
J. H. and Davidson, D. H.; Measuring Water Quality and Predicting Well
Impairment. JPT (1972).
- Bayona,
H. J.: "A Review of Well Injectivity Performance in Saudi Arabia's Ghawar
Field Seawater Injection Program," paper SPE 25531 presented at the SPE
1993 Middle East Oil Technical Conference and Exhibition, Bahrain.
- Chitwood,
G.B. and Coyle, W.R.: "New
Options to Eliminate Corrosion of Completion Equipment in Water Injection
Service," paper SPE 27853 presented at the 1994 SPE Western Regional
Meeting, Long Beach, CA, March 23-25.
- Coleman,
J. R. and McLelland, W. G.: "Produced Water Re-Injection; How Clean is
Clean?" paper SPE 27394
presented at the 1994 Symposium on Formation Damage Control, Lafayette,
Louisiana.
- Danyluk,
T. L., Chachula, R. C. and Solanki, S. C.: "Field Trial of the First
Desanding System for Downhole Oil/Water Separation in a Heavy-Oil
Application," paper SPE 49053 presented at the 1998 SPE Annual Technical
Conference and Exhibition, New Orleans, Louisiana.
- Davies,
D. R.; Lecture Notes in Production Technology, Heriot-Watt University. Edinburgh (2000).
- Donaldson,
E. C., Baker, B. A. and Carroll, H. B.: "Particle Transport in
Sandstones," paper SPE 6905
presented at the 1977 (56th) Annual Fall Technical Conference and
Exhibition of the SPE of AIME, Denver, Colorado.
- Fambrough,
J. D., Lane, R. H. and Braden, J. C.: "A Comprehensive Approach for
Stimulating Produced Water Injection Wells at Prudhoe Bay, Alaska," paper
SPE 28976 presented at the 1995 SPE International Symposium on Oilfield
Chemistry, San Antonio, Texas, February 14-175.
- Gruesbeck,
C. and Collins, R. E.: "Entrainment and Deposition of Fine Particles in
Porous Media," paper SPE
8430, SPEJ (1982).
- Herzig,
J. P., Leclerc, D. M. and Goff, P. L.: "Application to Deep
Filtration," Industrial and Engineering Chemistry (1970).
- King,
R. W. and Adegbesan, K. O.: "Resolution of the Principal Formation Damage
Mechanisms Causing Injectivity and Productivity Impairment in the Pemina
Cardium Reservoir," paper SPE 38870 presented at the 1997 SPE Annual
Technical Conference and Exhibition, San Antonio, Texas.
- Martins,
J. P., Murray, L. R., Clifford, P. J. G., McLelland, G., Hanna, M. F. and Sharp
jr., J. W.: "Long-Term Performance of Injection Wells at Prudhoe Bay: The Observed
Effects of Thermal Fracturing and Produced Water Re-Injection," paper SPE
28936 presented at the 1994 (69th) Annual Technical Conference and
Exhibition, New Orleans, LA.
- Meyers,
B.D., Daggett, L.P., and Cowden, J.R.:
"Pecos River Water Treatment for Water Injection, paper SPE 6883
presented at the 1977 Annual Fall Technical Conference and Exhibition of the
SPE of AIME, Denver, CO, October 9-12.
- Muecke,
T. W.: "Formation Fines and Factors Controlling their Movement in Porous
Media," SPE 7007, JPT (1979).
- Nassivera,
M. and Essel, A.: "Fateh
Field Sea Water Injection-Water Treatment, Corrosion and Scale Control,"
paper SPE 7765 presented at the 1979 SPEP Middle East Oil Technical Conference,
Manama, Bahrain, March 25-29.
- Nielsen,
M.F. and Bingham, S.:
"Increasing Availability - The Benefits of Introducing an
Integrated Corrosion Management System," paper SPE 50076 presented at the
1998 SPE Asia Pacific Oil and Gas Conference and Exhibition, Perth, Austraila,
October 12-14.
- Ohen,
H. A. and Civan, F.: "Predicting Skin Effects Due to Formation Damage by
Fines Migration," 1991 Production Operations Symposium, Oklahoma City,
Oklahoma.
- Ohen,
H. A., Nnabuihe, L., Felber, B. J., Ososanwo, D. and Holmgren, C. M.: "A
Systematic Laboratory Core and Fluid Analysis Program For The Design of Cost
Effective Treatment and Cleanup Guidelines for a Produced water Disposal
Scheme," paper SPE 35369 presented at the 1996 SPE 10th Symposium on IOR,
Tulsa, Oklahoma.
- Roque,
C., Chauveteau, C. and Renard, M.E.A.: "Mechanism of Formation Damage by
Retention of Particles Suspended in Injection Water," paper SPE 30110
presented at the 1995 European Formation Damage Conference, den Haague, The
Netherlands.
- Rosser,
H.R., Nasr-El-Din, H.a., Al-Shammari, N.A. and Al-Dhafeeri, A.M.: "Injection Water Treatment at the
Source: Biocide-Enhanced Corrosion
Inhibitor Squeeze Treatments of Water Supply Wells in a Central Arabia
Oilfield," paper SPE 50740 presented at the 1999 SPE International
Symposium on Oilfield Chemistry, Houston, Texas, February 16-19.
- Salma,
T.: "Cost Effective Removal
of Iron Sulfide and Hydrogen Sulfide from Water Using Acrolein," paper SPE 59708 presented at the 2000
SPE Permian Basin Oil and Gas Recovery Conference, Midland, Texas, March 21-23.
- Selmeczi,
J. G.: "Capture Mechanisms in Deep-Bed Filtration," Industrial
Water Engineering (1971).
- Sharma,
M. M., Pang, S., Wennberg, K. E. and Morgenthaler, L.: "Injectivity
Decline in Water Injection Wells: An Offshore Gulf of Mexico Case Study,"
paper SPE 38180 presented at the 1997 SPE European Formation Damage Conference,
The Hague, The Netherlands.
- Tang,
J.: "Injectivity Damage Caused by Re-Injection of Oily Produced
Water," Petroleum Society of CIM, Journal Article no. 82-33-55 (1982).
- Todd,
A. C.: Lecture Notes in Production Technology, Heriot-Watt University.
Edinburgh (1998).
- Vetter,
O. J., Karpa, V., M., S. and Veith, E.: "Particle Invasion into Porous
Medium and Related Injectivity Problems," paper SPE 16255 presented at the
1987 SPE International Symposium on Oilfield Chemistry, San Antonio, Texas.
- Wilkie,
D. I., Kennedy, W. L. and Tracy, K. F.: "Produced Water Disposal - A
Learning Curve in Yemen," paper SPE 35030 presented at the 1996 SPE
Formation Damage Symposium, Lafayette, Louisiana.
- Zhang,
N.: "Study of Permeability Alterations Associated with the Invasion of Oil
Droplets and Solid Particles in Produced Oily Water into Porous Media,"
Dept. of Petroleum Engineering, Supervisor Prof. Adrian Todd, (1994).
- Zhang,
N. S., Somerville, J. M. and Todd, A. C.: "An Experimental Investigation
of the Formation Damage Caused by Produced Oily Water Injection," paper
SPE 26702 presented at the 1993 Offshore European Conference, Aberdeen,
September 7-10.




